Proteinopati


Proteinfejlfoldnings-sygdom, proteinopati eller proteopati (eng. protein misfolding disease, PMD, eller protein conformational disorder, PCD) er en klasse af sygdomme, hvor proteiner bliver strukturelt ændret fra det normale for derved at forstyrre, hæmme eller afspore de normale funktioner i celler, væv og organer.[1][2][3][4]
Proteinopatierne omfatter neurodegenerative sygdomme, bl.a. Alzheimers sygdom, Parkinsons sygdom, multipel systematrofi, polyQ-sygdomme bl.a. Huntingtons sygdom og andre polytrinukleotid-sygdomme, som Creutzfeldt-Jakobs sygdom og andre prionsygdomme, amyloidose og en lang række andre lidelser, se liste.[5]
Den biokemiske mekanisme
Når proteiner ikke folder til deres normale konfiguration, mister de deres normale funktion, og hvis de ikke efterfølgende nedbrydes men oplagres, blive de giftige.
I næsten alle kendte tilfælde er den sygdomsfremkaldende molekylære ændring en øgning af et proteins beta-sheets. I nogle proteinopatier har det sygdomsfremkaldende protein vist sig at folde til flere 3-dimensionelle former; disse variante strukturer kan have forskellige konformationer med patogene egenskaber, som er blevet mest grundigt undersøgt med hensyn til prionsygdommene.
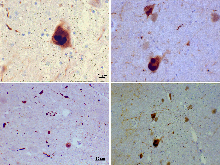
Alzheimers sygdom og andre lidelser er karakteriseret ved akkumulering af tau-proteinet i fibrillære aggregater og betegnes som en tauopati. I den aldrende hjerne kan flere proteopatier overlappe hinanden. For eksempel, ud over tauopati og Aβ-amyloidose (som eksisterer sideløbende som patologiske nøgletræk ved Alzheimers sygdom), har mange Alzheimerpatienter samtidig synucleinopati (Lewy-legemer) i hjernen.
Sandsynligheden for, at proteinopati vil udvikle sig, øges af visse risikofaktorer, der fremmer selvsamlingen af proteinmolekyler. Disse omfatter mutationer der destabiliserer proteinets primære aminosyresekvens, jf. polyQ-sygdomme. Andre risikofaktorer er post-translationelle modifikationer som hyperphosphorylering og en stigning i produktionen af et protein eller et fald i dets clearance.
De mest undersøgte proteiner, der kan danne sygdomsfremkaldende aggregater er følgende:
- serum amyloid A, SAA (serum amyloid A)[6][7]Vs. Science 2022]</ref>
- prion protein, PP[8]
- serum amyloid A, SAA (serum amyloid A)[6][7]
- synuclein, alpha-synuclein[9]
- tau-protein [6][10]
- TDP-43, TAR DNA-binding protein 43 [11]
Mange andre proteiner kan undergå de strukturelle ændringer, som medfører sygdomsfremkaldende aggregater, se List of proteinopathies.
Henvisninger
- ^ Protein Misfolding and Degenerative Diseases. Scitable by Nature Education 2010
- ^ Proteinopathy. Science Direct
- ^ Proteinopathy. Science Direct 2018
- ^ Protein-misfolding diseases and chaperone-based therapeutic approaches. FEBS Journal 2006
- ^ Protein Misfolding and Human Disease. Annual Review of Genomics and Human Genetics 2006
- ^ a b c New Theory Tries to Explain The Cause of Alzheimer’s Disease. Mind the Graph 2021
- ^ a b Serum amyloid A – a review. Molecular Medicine 2018
- ^ Cellular and Molecular Mechanisms of Prion Disease. Annual Review of Pathology 2019
- ^ Insights into the molecular mechanism of amyloid filament formation: Segmental folding of α-synuclein on lipid membranes. Science Advances 2021
- ^ Amyloid fibril structures of tau: Conformational plasticity of the second microtubule-binding repeat. Science 2023
- ^ The role of TDP-43 propagation in neurodegenerative diseases: integrating insights from clinical and experimental studies. Nature 2023
Medier brugt på denne side
Forfatter/Opretter: Suraj Rajan, Licens: CC BY-SA 3.0
Photomicrograph of regions of substantia nigra in a Parkinson's patient showing Lewy bodies and Lewy neurites in various magnifications. Top panels show a 60-times magnification of the alpha synuclein intraneuronal inclusions aggregated to form Lewy bodies. The bottom panels are 20 × magnification images that show strand-like Lewy neurites and rounded Lewy bodies of various sizes. Neuromelanin laden cells of the substantia nigra are visible in the background. Stains used: mouse monoclonal alpha-synuclein antibody; counterstained with Mayer's haematoxylin.
Photomicrograph of a section through the cerebral cortex of a patient with Alzheimer's disease. The section was immunostained with an antibody to the protein Abeta (brown) and counterstained with hematoxylin (blue). 10X microscope objective.
Forfatter/Opretter: Mark R Cookson, Licens: CC BY 2.0
Events in α-synuclein toxicity. The central panel shows the major pathway for protein aggregation. Monomeric α-synuclein is natively unfolded in solution but can also bind to membranes in an α-helical form. It seems likely that these two species exist in equilibrium within the cell, although this is unproven. From in vitro work, it is clear that unfolded monomer can aggregate first into small oligomeric species that can be stabilized by β-sheet-like interactions and then into higher molecular weight insoluble fibrils. In a cellular context, there is some evidence that the presence of lipids can promote oligomer formation: α-synuclein can also form annular, pore-like structures that interact with membranes. The deposition of α-synuclein into pathological structures such as Lewy bodies is probably a late event that occurs in some neurons. On the left hand side are some of the known modifiers of this process. Electrical activity in neurons changes the association of α-synuclein with vesicles and may also stimulate polo-like kinase 2 (PLK2), which has been shown to phosphorylate α-synuclein at Ser129. Other kinases have also been proposed to be involved. As well as phosphorylation, truncation through proteases such as calpains, and nitration, probably through nitric oxide (NO) or other reactive nitrogen species that are present during inflammation, all modify synuclein such that it has a higher tendency to aggregate. The addition of ubiquitin (shown as a black spot) to Lewy bodies is probably a secondary process to deposition. On the right are some of the proposed cellular targets for α-synuclein mediated toxicity, which include (from top to bottom) ER-golgi transport, synaptic vesicles, mitochondria and lysosomes and other proteolytic machinery. In each of these cases, it is proposed that α-synuclein has detrimental effects, listed below each arrow, although at this time it is not clear if any of these are either necessary or sufficient for toxicity in neurons. Cookson Molecular Neurodegeneration 2009 4:9 doi:10.1186/1750-1326-4-9


