Mæslingevirus

Mæslingevirus eller mæslinge morbilivirus, MV (en. Measles morbillivirus), tidligere kaldt mæslingevirus (MeV) forårsager mæslinger. Mennesket er den naturlige vært, og der kendes intet reservoir for denne virus i noget dyr.
Mæslingevirus er beslægtet med den nu-udryddede kvægpest-virus, RPV (rinderpest virus).
Generelt
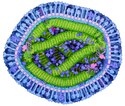
MV er en membrankappet morbilivirus med et genom af -ssRNA (negativt polariseret enkeltstrænget RNA) med 15.896 baser og 6 gener, der koder for 8 proteiner:
- Fusionprotein [2]
- Matrixprotein
- RNA dependant RNA polymerase, RdRp
- Phosphoprotein + V-protein + C-protein [3]
- Nucleoprotein.[1]
Receptorer for mæslingevirus
Mæslingevirus binder sig med overfladeproteinet hemagglutinin til receptorer på overfladen af værtsorganismens celler:
- CD46 som udtrykkes på næste alle menneskets celler
- CD150 eller SLAM (signaling lymphocyte activation molecule) som er på B- og T-celler og andre immunceller
- nectin-4 som er et adhæsionsmolekyle på overfladen af mange af menneskets celler inklusivt nerveceller.
Vaccine
Der er 23 undertyper af mæslingevirus men kun en serotype. Med en vaccine fremstillet af svækkede mæslingevirus opnås der immunitet. Vaccinen er en kombinationsvaccine, MFR-vaccine, mod mæslinger, fåresyge og røde hunde.
Henvisninger
|
Medier brugt på denne side
Forfatter/Opretter: David Goodsell, Licens: CC BY 4.0
Cross section through measles virus. The virus is enveloped by a lipid membrane (light magenta) studded with many hemagglutinin and fusion proteins (outermost proteins in blue), which together bind to human cells and enter them. The viral genome is a strand of RNA (yellow) protected by nucleoproteins (green). RNA-dependent RNA polymerase (bright magenta) copies the RNA once the virus infects a cell, assisted by the largely-disordered phosphoprotein (purple strands connecting the polymerase to the nucleoprotein). Matrix protein (turquoise) helps the virus bud from infected cells. Several human proteins, such as actin and integrins, are also caught in the budding virus (shown in purple). This painting was created for the Molecule of the Month on Measles Virus Proteins.
This thin-section transmission electron micrograph (TEM) revealed the ultrastructural appearance of a single virus particle, or “virion”, of measles virus. The measles virus is a paramyxovirus, of the genus Morbillivirus. It is 100-200 nm in diameter, with a core of single-stranded RNA, and is closely related to the rinderpest and canine distemper viruses. Two membrane envelope proteins are important in pathogenesis. They are the F (fusion) protein, which is responsible for fusion of virus and host cell membranes, viral penetration, and hemolysis, and the H (hemagglutinin) protein, which is responsible for adsorption of virus to cells.
There is only one antigenic type of measles virus. Although studies have documented changes in the H glycoprotein, these changes do not appear to be epidemiologically important (i.e., no change in vaccine efficacy has been observed).
Prior to 1963, almost everyone got measles; it was an expected life event. Each year in the U.S. there were approximately 3 to 4 million cases and an average of 450 deaths, with epidemic cycles every 2 to 3 years. More than half the population had measles by the time they were 6 years old, and 90 % had the disease by the time they were 15. This indicates that many more cases were occurring than were being reported. However, after the vaccine became available, the number of measles cases dropped by 98 % and the epidemic cycles drastically diminished.
Measles virus is rapidly inactivated by heat, light, acidic pH, ether, and trypsin. It has a short survival time (<2 hours) in the air, or on objects and surfaces.