Kinetisk gasteori
Kinetisk gasteori er en fysisk model, der forklarer gassers makroskopiske egenskaber såsom tryk og temperatur ved at beskrive dem som bestående af partikler i bevægelse. Hvis partiklerne ikke interagerer med hinanden og er i termisk ligevægt kan det vises, at partiklernes hastigheder er fordelt jf. Maxwell-Boltzmann-fordelingen. Derved kan den kinetiske gasteori bruges til at udlede idealgasligningen.[1]
Kildehenvisninger
- ^ Blundell, Stephen J.; Blundell, Katherine M. (2006). "Part II Kinetic theory of gases". Concepts in Thermal Physics (engelsk) (1. udgave). Oxford University Press. p. 45. ISBN 978-0-19-856770-7.
| Spire Denne artikel om fysik er en spire som bør udbygges. Du er velkommen til at hjælpe Wikipedia ved at udvide den. |
|
Medier brugt på denne side
Nuclear physics
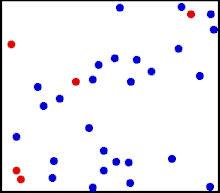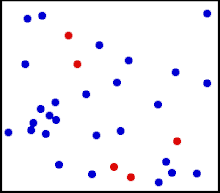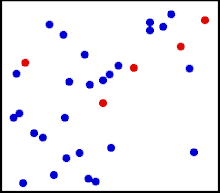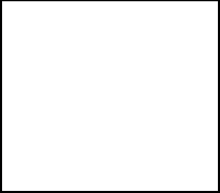
Motion of gas molecules.
The randomized thermal vibrations of fundamental particles such as atoms and molecules—gives a substance its “kinetic temperature.” Here, the size of helium atoms relative to their spacing is shown to scale under 1950 atmospheres of pressure. These room-temperature atoms have a certain, average speed (slowed down here two trillion fold). At any given instant however, a particular helium atom may be moving much faster than average while another may be nearly motionless. The rebound kinetics of elastic collisions are accurately modeled here. If the velocities over time are plotted on a histogram, a Maxwell-Boltzmann distribution curve will be generated. Five atoms are colored red to facilitate following their motions.
Note that whereas the relative size, spacing, and scaled velocity of the atoms shown here accurately represent room-temperature helium atoms at a pressure of 1950 atmospheres, this is a two-dimensional scientific model; the atoms of gases in the real world aren’t constrained to moving in two dimensions in windows precisely one atom thick. If reality worked like this animation, there would be zero pressure on the two faces of the box bounding the Z-axis. The value of 1950 atmospheres is that which would be achieved if room-temperature helium atoms had the same inter-atomic separation in 3-D as they have in this 2-D animation.